Aromatics Transalkylation
reference information
This process is characterized by simultaneous disproportionation of several hydrocarbons, e.g., toluene and trimethylbenzenes.
During reaction of arenes transalkylation the migration of alkyl group from the molecule of one aromatic compound to the molecule of another hydrocarbon takes place. For example, toluene forms benzene and xylene.
In addition, the reaction of m-xylene and C9 aromatic hydrocarbons isomerization takes place.
Approximate material balances (in % wt.) of disproportionation and transalkylation processes with recycle of unreacted C7 and C9 aromatic hydrocarbons correspondingly are shown below:
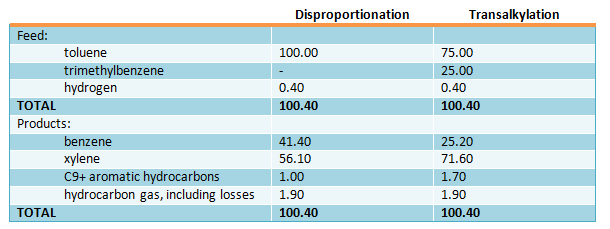
The processing scheme may be used depending on the requirements to the aromatic hydrocarbons, and also toluene and trimethylbenzenes (С9Н12) resources using isomerization, disproportionation and transalkylation processes over zeolite catalyst without noble metal. The hydrodealkylation process is also possible to apply.
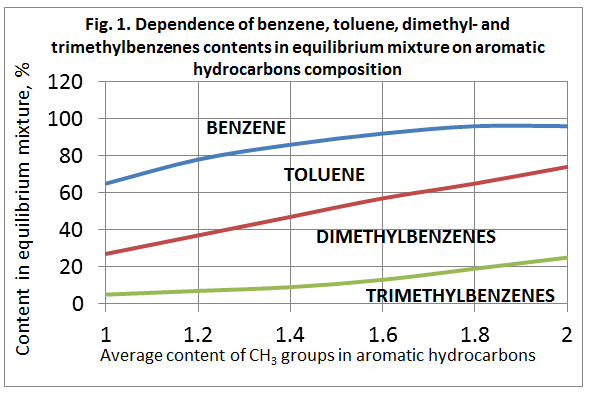
Thermodynamic dependencies of methylbenzenes disproportionation and transalkylation reactions have been studied by many authors. Fig. 1 shows the dependency of benzene, toluene, dimethyl- and trimethylbenzenes contents at the temperature of 477 °C. The equilibrium was stated to change insignificantly in the case of temperature variation in the range of 300-600 °C.
Shown data allow to calculate the equilibrium mixture composition at 477 °C, that is received trough disproportionation and transalkylation reactions as a function of concentrations in initial mixture of aromatic hydrocarbons – C7 and C9 methylbenzenes. Data of equilibrium mixture composition as a function of feed composition are shown below:
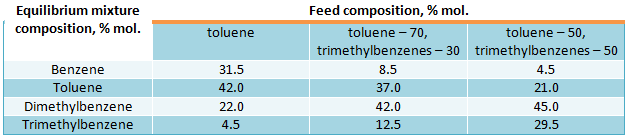
Using toluene as initial feed maximal depth of its “once through” conversion cannot exceed 58%.
The ratio of produced benzene and xylenes may be controlled by changing the initial feed composition that significantly increases the flexibility of disproportionation unit. Chlorinated aluminum, hydrogen fluoride, boron trifluoride, alumina, and amorphous and crystalline aluminosilicates have been studied as disproportionation catalysts. In petrochemical industry only heterogeneous catalysts have been applied. Disproportionation process may be carried out at the atmospheric pressure and under hydrogen pressure.
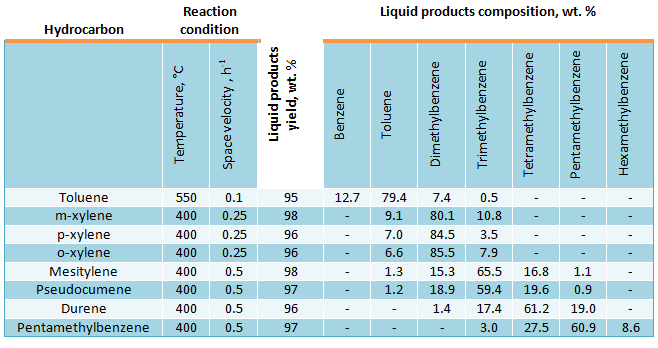
Methylbenzenes disproportionation reaction rate over amorphous aluminosilicate catalyst at the atmospheric pressure is described by the second-order equation. If toluene disproportionation reaction rate is taken at 400°C, then kinetic constants of C8-C11 methylbenzenes disproportionation will be equal: orthoxylene – 10, metaxylene – 12, paraxylene – 16, pseudocumene – 30, mesitylene – 30, durene – 52, pentamethylbenzene – 17, i.e. the lowest disproportionation reaction rate will be observed for toluene. If amount of methyl groups in benzene hydrocarbons rises, the disproportionation reaction rate increases. However, this is not true in all cases– in passing from tetramethylbenzene to pentamethylbenzene the reaction rate decreases that is probably connected with three-dimensional configuration of pentamethylbenzene molecule. Results of C7-C11 methylbenzenes disproportionation are shown in table below. Resultant composition of di-, tri- and tetramethylbenzenes in disproportionation reaction is close to thermodynamical equilibrium.
Results of C7-C11 methylbenzenes disproportionation over aluminosilicate catalyst:
Toluene and pentamethylbenzene transalkylation reaction over aluminosilicate catalyst was proposed to obtain xylenes with enhanced quantity of paraxylene and tetramethylbenzenes (US Patent No. 3350469, 1967). Transalkylation is carried out at the temperature of 315 °C and pressure of 1.0 h-1. Using 77.9 wt. % toluene and 22.1 wt. % pentamethylbenzene as an initial feed, 95 wt. % liquid reaction products are received; in this case coke is contained on the catalyst in amount of 0.3 wt. %. Liquid products composition is the following (in wt. %): benzene – 0.2; toluene – 62.5; dimethylbenzenes – 16.5; trimethylbenzenes – 3.8; tetramethylbenzenes – 9.5; pentamethylbenzene – 7.4; hexamethylbenzene – 0.1. Conversion depth of toluene is 24%, pentamethylbenzene – 68.5%. Selectivity to formation of di- and tetramethylbenzenes is 79%. Composition of resultant dimethylbenzenes is the following (in wt. %): paraxylene – 63.5; metaxylene – 30.5; orthoxylene – 6.0. Considerably higher concentration of paraxylene than thermodynamical equilibrium concentration apparently results from blocking of active sites of isomerization catalyst by pentamethylbenzene.
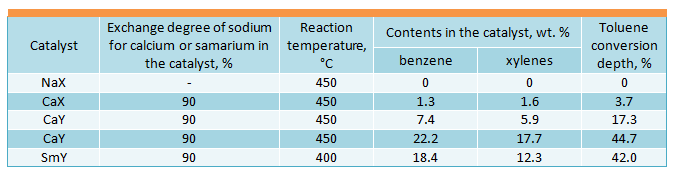
Taking into account the insufficiently high activity of amorphous aluminosilicates, the disproportionation reactions were conducted in the presence of crystalline aluminosiliates – zeolites (Morita Y., J. Japan Petrol. Inst., 1967, vol. 10, No. 7, pp. 429-435, Morita Y., J. Chem. Soc. Japan, 1967, vol. 70, No. 8, pp. 1363-1372). Studies of different cationic forms of X- and Y-types zeolites showed that their multivalent forms are catalytically active in disproportionation reaction of alkylaromatic hydrocarbons. The X-type zeolites (molar ratio SiО2 : Аl2О3 = 2,5) and Y-type zeolites (molar ratio SiО2 : Аl2О3 = 5) with different degree of exchange of sodium ion for calcium ion have been studied in detail (Isakov Ya.I., Minachev Kh.M., “Petroleum Chemistry”, 1967, vol. 7, No. 4, pp. 561-568; Isakov Ya.I., Minachev Kh.M., “Petroleum Chemistry”, 1967, vol. 10, No. 6, pp. 805-812). It was stated as a result that in the case of increase of exchange degree of sodium for calcium in X-type and Y-type zeolites, the catalyst activity rises. In both cases the best results were obtained with exchange degree of 90 %.
Y-type zeolites were found to be more active than X-type zeolites. Examined catalysts were in the following sequence according to activity: SmY > HY >CaY >СаХ. NaX and NaY zeolites were not active.
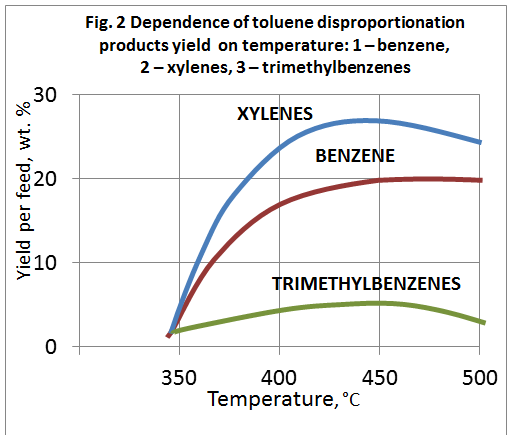
Toluene disproportionation results over different zeolite catalysts at feed LHSV 0.3 h-1 are shown below (Isakov Ya.I., Minachev Kh.M., “Petroleum Chemistry”, 1967, vol. 7, No. 4, pp. 561-568; Isakov Ya.I., Minachev Kh.M., “Petroleum Chemistry”, 1967, vol. 10, No. 6, pp. 805-812).
Effect of process parameters to toluene disproportionation over CaY catalyst was studied in the work of Mortikov E.S., etc., “Petroleum refining and petroleum chemistry”, 1972, No. 2, pp. 31-34. To improve strength and formability 30% of alumina is introduced into CaY zeolite. Study of temperature effect on disproportionation reaction showed that toluene conversion reaction begins to take place effectively at the temperature of 350 °С, pressure of 1.5 MPa (15 kgf/cm2), feed space velocity of 0.6 h-1. Maximal disproportionation products yield was observed at 450-500°C (fig. 2). Composition of resultant xylenes was the following (in wt. %): paraxylene – 24; metaxylene – 50; orthoxylene – 26; the trimethylbenzenes composition (in wt. %): mesitylene – 21; pseudocumene – 69; hemimellitene – 10.
Dependence of disproportionation products yield on duration of catalyst operation at 450 °C and feed space velocity 1.0 h-1 is shown on fig. 3. Increase of duration of catalyst operation is accompanied by decrease of disproportionation products yield, especially at high pressure. In the case if the process is carried out at the atmospheric pressure, the disproportionation products yield is nearly on the same level.
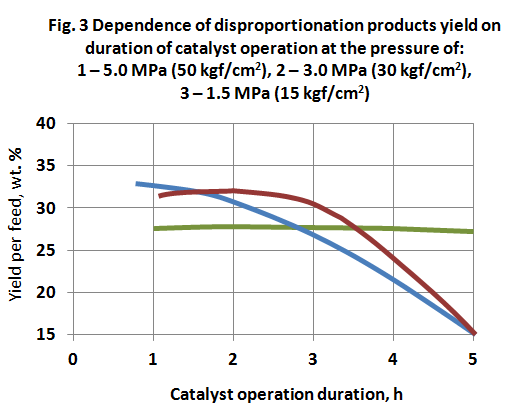
As a result of performed works the following toluene disproportionation process conditions over CaY catalyst are recommended: temperature of 450 °С, feed space velocity of 0.6 h-1, pressure of 1.5 MPa (15 kgf/cm2), operation duration of 5 h, after that it is necessary to carry out oxidative regeneration of the catalyst. In this conditions the products yield is the following (in wt. %): benzene – 20-22; xylenes – 30-31; trimethylbenzenes – 4-5; coke – 1-3; gas products – 1-2.
Activity of CaY zeolite catalyst in disproportionation reaction may be improved by addition of small quantities of oxygen and oxygenates to the feed (US Patent 3437709, 1969). So, at 425 °C, space velocity 0.33 h-1 and duration of catalyst operation 7 h, the toluene conversion depth was 7.3%. After addition of 2 wt. % of oxygen to the feed it increased up to 29.7%, and after water addition (0.45 wt. % 02) — up to 21.1%.
RZH catalyst was studied in toluene disproportionation (in liquid phase process) at 290 °c, 4.5 MPa (45 kgf/cm2) and 1.4 h-1 (US Patent 3377400, 1968; Grandio P., et al., Preprints General Papers Division of Petrol. Chem. Inc., 1971, vol. 16, No. 3, pp. 78-88). This catalyst is also applied in xylenes isomerization process, but isomerization reaction is carried out at lower temperature. Application of liquid phase allowed to considerably lengthen the non-regenerative service life of the catalyst without hydrogen using in the process. Toluene disproportionation results over RZH catalyst at the given conditions are shown below (in wt. %) (Grandio P., et al., Hydrocarb. Proc., 1972, vol. 51, No. 8, pp.85-86):
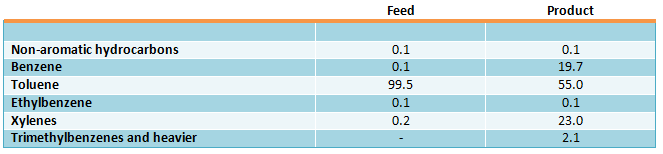
Liquid phase disproportionation process is characterized by high selectivity of toluene conversion — trimethylbenzenes yield is about 2 %.
Disproportionation process under hydrogen pressure allows to decrease carbon deposition on the catalyst and increase duration of non-regenerative catalyst operation.
Study of the catalysts in transalkylation reaction of aromatic hydrocarbons mixture (84.8 mol. % of toluene and 15.2 mol. % trimethylbenzenes) under pressure of 3 MPa (30 kgf/cm2), space velocity of 1 h-1 and H2 : HC molar ratio = 8, showed that decationated mordenite was found the most active and selective catalyst (Belgian Patent 716016, 1969):
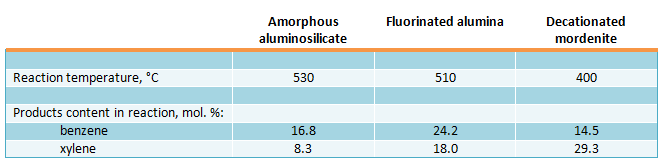
In the presence of amorphous aluminosilicate and fluorinated alumina the dealkylation reaction takes place in the great extent, besides transalkylation reaction.
The following catalysts were proposed to carry out the disproportionation reaction under hydrogen pressure: platinum-rhenium alumina (US Patent 3646236, 1972), nickel fluorinated aluminosilicate (US Patent 3651162, 1972), molybdenum fluorinated alumina (US Patent 3663636, 1972), aluminum fluoride-supported decationated mordenite (Yoshima T., Bull. Japan Petrol. Inst., 1970, vol. 12, pp. 106-111; Japan Patent 20210, 1972), etc.
Behavior of toluene disproportionation reaction under hydrogen pressure was studied over zeolite catalysts (Bursian N.R., et al., “Chemistry and Technology of Fuels and Oils”, 1974, No. 5, pp. 8-10).
The following products yields (in wt. %) were obtained in disproportionation process of toluene, containing 0.5 wt. % of benzene, at pressure of 4 MPa (40 kgf/cm2), space velocity of 1.0 h-1, H2 : HC molar ratio of 10 : 1 and 500 °С:
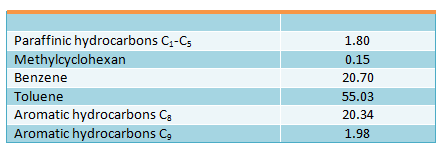
Aromatic hydrocarbons C8 composition was the following (in wt. %): ethylbenzene – 0.8; paraxylene – 23.4; metaxylene – 52.4; orthoxylene – 23.4. Selectivity of toluene disproportionation process to benzene and aromatic hydrocarbons C8 is 93%. The applied catalyst possessed slightly improved hydrogenating properties, as about 2 wt. % of paraffinic and naphthenic hydrocarbons were formed.
Results of toluene and trimethylbenzenes transalkylation reaction over decationated mordenite at the temperature of 410 °C, pressure of 3 MPa (30 kgf/cm2), space velocity of about 0.7 h-1 and hydrogen flowrate of 800 m3/m3 per feed are shown below (Belgian Patent 716016, 1969):
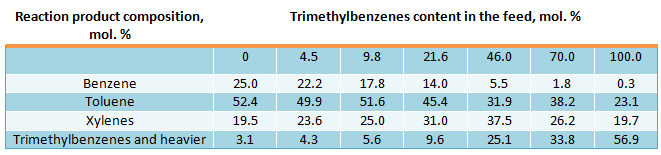
Depending on the trimethylbenzenes content in the feed the ratio of xylenes to benzene yields can vary from 0.78 to 60. Using mixture, consisting of 90.2 mol. % toluene and 9.8 mol. % trimethylbenzenes, as a feed, the same ratio of toluene to trimethylbenzene in reaction products was obtained as in the feed, i.e. in this case the process may be conducted with toluene and trimehylbenzenes recycling; at this the constant ratio of xylene and benzene yields will be observed, that equals to 1.4. If it is required to increase xylenes to benzene yields, it is necessary to use additional quantity of trimethilbenzenes from the other sources.
Xylenes has the following composition, resultant from toluene disproportionation and toluene and trimethylbenzenes transalkylation reactions (in mol. %): ethylbenzene – 0.8-1.1; paraxylene – 21.7-23.7; metaxylene – 51.0-54.4; orthoxylene – 22.8-24.5. Xylenes concentration is close to thermodynamically equilibrium concentration of dimethylbenzenes. It results from isomerization activity of decationated mordenite. So, the composition of the resultant xylenes in disproportionation reaction after addition of 20 vol. % of metaxylene to toluene was the following (in mol. %): ethylbenzene – 1.0; paraxylene – 23.0; metaxylene – 52.0; orthoxylene – 24.0.
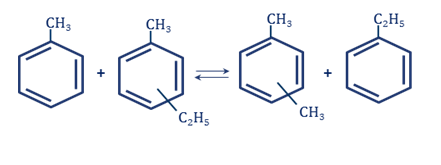
In transalkylation processes to increase xylene to benzene yield the aromatic hydrocarbons C9, produced in catalytic reforming of gasolines, are involved into reaction. Among these aromatic hydrocarbons C9 besides trimethylbenzenes the great amount of ethyltoluenes is contained. In this case the ethylbenzene content in xylenes, produced in the transalkylation process, will be slightly increased. It is connected with reaction chemistry:
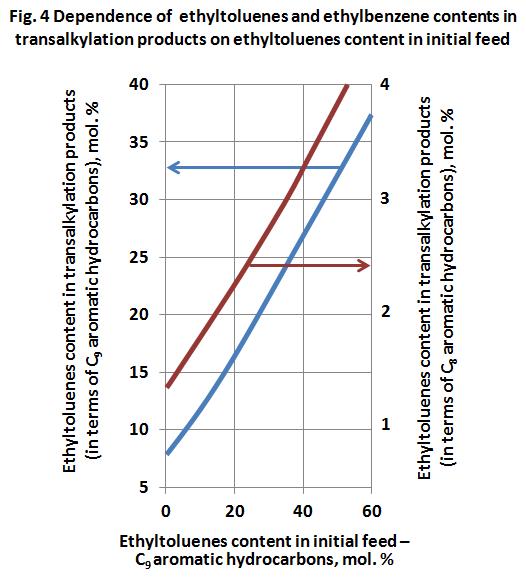
Effect of ethyltoluenes content in transalkylation feed to the composition of generated aromatic hydrocarbons C8 and C9 was studied on the feed, containing 96 mol. % of toluene and 4 mol. % of aromatic hydrocarbons C9. Ethyltoluenes content in the feed — aromatic hydrocarbons C9 — was varied from 0 to 40 mol. %. Fig. 4 shows the dependence of ethyltoluenes and ethylbenzene contents in transalkylation products on ethyltoluenes content in the feed — aromatic hydrocarbons C9 (Morita Y., J. Japan Petrol. Inst., 1967, vol. 10, No. 7, pp. 429-435). For example, if aromatic hydrocarbons C9 contain 15 mol. % of ethyltoluenes, the amount of ethylbenzene in produced xylenes will be equal to 2 mol. %. This figure shows the ethyltoluenes content in aromatic hydrocarbons C9 generated in reaction. This data indicate that ethyltoluenes concentration is decreased in the products, i. e. ethyltoluenes are not accumulated in operation with aromatic hydrocarbons C9 recycling.
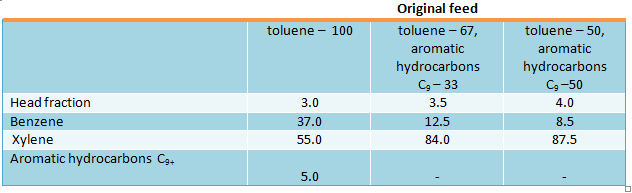
Process for disproportionation and transalkylation of aromatic hydrocarbons called Xylene Plus was developed by Sinclair Oil Corp. (USA) (Verdol I.A., Oil a. Gas J., 1969, vol.67, №23, pp. 63-66). Aluminosilicate zeolite catalyst based on rare-earth X or Y zeolite (rare-earth oxides content is up to 5 wt.%) is used this process (US patent 3551509, 1970). Toluene disproportioning over this catalyst proceeds at 540 °С and 0.9 h-1. Toluene “once-through” conversion is 33.6%. The process has the following selectivity: benzene production – 55%, xylene – 35%, reaction by-products – 10%. The following product yield (wt.%) is obtained under recycle of unreacted toluene (Feed/recycle ratio is 1:0.664): benzene – 46.8, aromatic hydrocarbons C9+ - 3.9, gas – 3.4, coke – 4.6 (US patent 3551509, 1970).
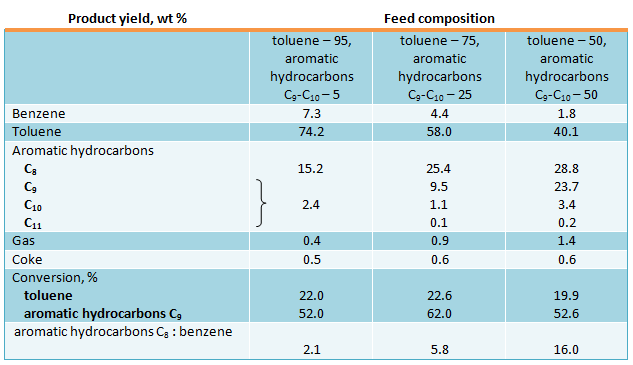
Please, see below the results of toluene and С9-С10 aromatic hydrocarbons transalkylation over this zeolite catalyst with the following feed composition (wt.%): n-propylbenzene – 0.36, ethyl toluenes – 10.71, trimethylbenzenes – 68.20, diethylbenzenes – 2.65, dimethylethylbenzenes – 14.06, tetramethylbenzenes – 2.88, aromatic hydrocarbons C11+ - 1.13 at 500 °С and 1.6 h-1 (US patent 3551509, 1970):
Xylene Plus process is performed under atmospheric pressure over dynamic-bed granulated catalyst in a system with separate reactor and regenerator. Operating modes of the commercial units are not disclosed. Liquid products composition of the recycle process is the following (vol%) (Verdol I.A., Oil a. Gas J., 1969, vol.67, №23, pp. 63-66):
Xylene composition (wt %): ethyl benzene 1; p-xylene 25; m-xylene 50; o-xylene 24.
Xylene Plus process makes it possible to involve С10 и С11 aromatic hydrocarbons into transalkylation reaction, as the process is not limited by coke formation on the catalyst. The process is rather flexible and allows using different cuts of monocyclic aromatic hydrocarbons produced on oil refining and petrochemical units.
Toray Industries Inc. (Japan) developed process of disproportionation and transalkylation of aromatic hydrocarbons under hydrogen pressure, which is called Tetorey (Iwamura T., Otani S., Sato M., Bull. Japan Petrol Inst., 1971, vol.13, pp. 116-122; Otani S. et al., Japan Chem. Quaterly, 1968, vol.4, pp. 16-18; Hydrocarb. Proc., 1969, vol. 48, №11, p. 155; Oil a. Gas J., 1969, vol.67, №33, p. 80; Otani S., Chem. Eng., 1970, vol.77, №16, pp. 118-120; Otani S. et al., Japan Petrol Inst., 1970, vol.13, №4, pp. 282-285; Europ. Chem. News, 1970, vol.17, №413, p.4). The catalyst is not disclosed; it is supposed to be dealuminated zeolite with the pore entrance of 4-5 Å (Patent of Belgium 724845, 1969) of H-mordenite type (US patent 3651162, 1972). Catalyst service cycle is 10 months, the total service life is over two years. Main process flow diagram is depicted in Figure 5 (Iwamura T., Otani S., Sato M., Bull. Japan Petrol Inst., 1971, vol.13, pp. 116-122).
Fig. 5 Main process flow diagram of disproportionation and transalkylation unit
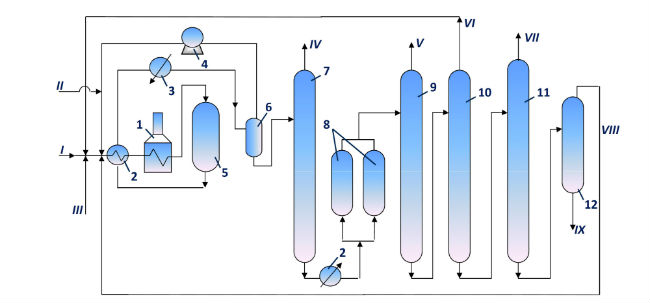
1 — tube furnace; 2 — heat exchangers; 3 — cooler; 4 — hydrogen gas recycle compressor; 5 — reactor; 6 — HP gas separator; 7 — Stabilizer; 8 — clay treatment adsorbers; 9 — benzene column; 10 — toluene column; 11 — xylene column; 12 — trimethylbenzene column.
I — feed — toluene; II — hydrogen; III — С9 aromatic hydrocarbons; IV — stabilization products; V — benzene; VI — toluene recycle; VII — xylene; VIII —С9 aromatic hydrocarbons recycle; IX — aromatic hydrocarbons С10+.
Original feed – toluene – is mixed with recycle hydrogen gas, then it is heated in furnace and is fed to reactor. Reaction products are separated in HP gas separator. Gas phase goes to recycle compressor suction, and liquid phase is stabilized and treated with clay. Further the products are rectified to recover benzene, toluene, xylenes, and trimethylbenzenes. Benzene and xylene recovered from the liquid products are commercial products. Toluene and trimethylbenzenes are mixed with the feed. In order to increase xylene production, a mixture of С9 aromatic hydrocarbons produced by catalytic reforming may be added to the feed.
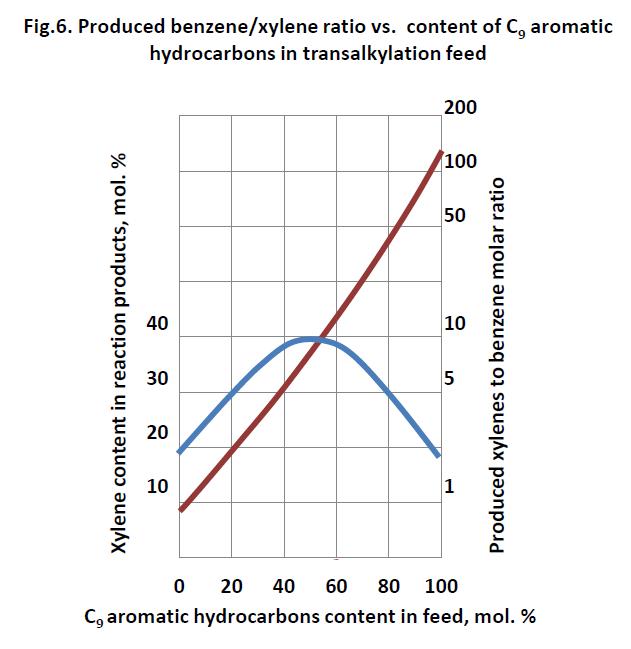
Process operating conditions are the following: 3 MPa (30 kgf/cm2); 400-450 °С; H2:HC, mole/mole 6-10 : 1; space velocity about 1.0 h-1; H2 content in recycle H2 gas; not less than 70 mol. % (Iwamura T., Otani S., Sato M., Bull. Japan Petrol Inst., 1971, vol.13, pp. 116-122). The unit is able to produce benzene and xylenes in the ratio from 0.8 : 1 to 1 : 10, subject to C9 aromatic hydrocarbons content in the feed (Fig. 6). If C9 aromatic hydrocarbons content in feed equals to 4 mol.%, then benzene/xylene ratio is 1. Maximum xylene content in reaction product is observed at 50 mol.% of C9 aromatic hydrocarbons content in the feed.
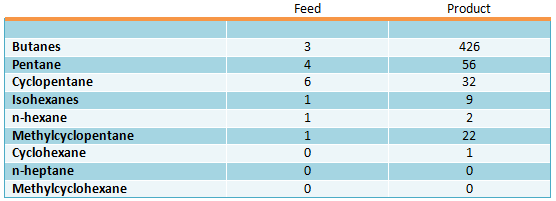
Small hydrogenating activity of the catalyst is the specific feature of Tetorey process – side reactions of aromatic hydrocarbons saturation are minimal. Information on composition of paraffinic and naphthenic hydrocarbons in feed produced during toluene disproportioning (mol. ppm) (Iwamura T., Otani S., Sato M., Bull. Japan Petrol Inst., 1971, vol.13, pp. 116-122):
Benzene forms azeotrope mixtures with paraffinic and naphthenic hydrocarbons boiling in the range of 62-100 °С. Reaction products contain just 25 ppm of hydrocarbons able to form azeotrope mixtures with benzene. Therefore, it can be recovered by simple rectification.
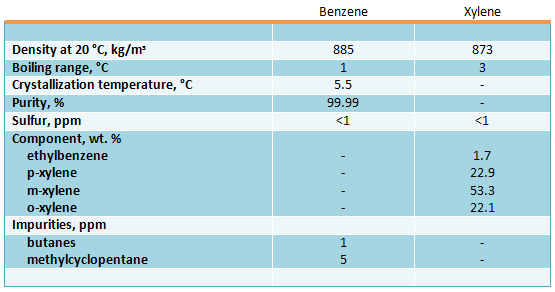
Properties of benzene and xylene produced by disproportioning are the following:
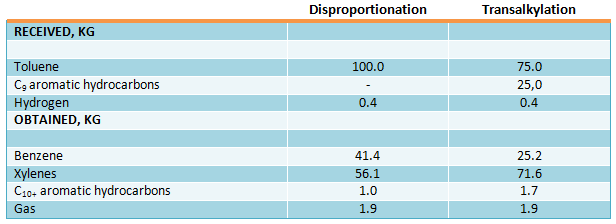
Material balance of disproportionation and transalkylation processes with recycle of unreacted С7 and С9 aromatic hydrocarbons is presented in the table below. Recycle of produced С9 aromatic hydrocarbons took place in toluene disproportioning process. Reactor feed composition (mol.%): toluene - 96, С9 aromatic hydrocarbons – 4 (Iwamura T., Otani S., Sato M., Bull. Japan Petrol Inst., 1971, vol. 13, pp. 116-122).
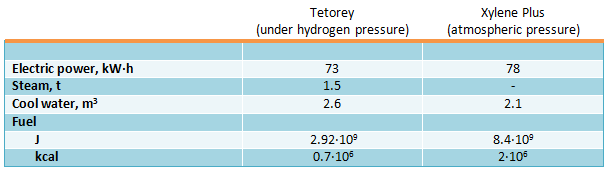
Tetorey process is used on disproportioning and transalkylation commercial unit in Kawasaki (Japan) with toluene production capacity of 70 KTA, and Xylene Plus process is applied on a commercial unit in Houston (Texas, USA) with feed capacity of 120 KTA. Consumption parameters of toluene disproportionation processes per 1 ton of original feed are as follows (Verdol I.A., Oil a. Gas J., 1969, vol.67, №23, pp. 63-66; Hydrocarb. Proc., 1972, vol.51 №8, pp. 85-86):
Information of this chapter is given exclusively for a reference purpose. You can find information about SIE Neftehim, LLC's products and services in Developments and Services chapters.










