C5-C6 Fractions Isomerization
Pentane-hexane isomerization process and catalysts
reference information
Isomerization is the process by which one isomer is transformed into another one. 
Isomerization leads to production of a compound with another atomic (group) arrangement but composition and molecular weight do not change. In literature isomerization is often called rearrangement, sometimes these are name reactions (isomerization processes) according to the tradition.
Isomerization process is aimed at production of high-octane components of commercial gasoline from low-octane oil fractions by structural change of carbon skeleton.
Isomerization of gasoline. Historical information (History Background)
The term isomery was introduced into organic chemistry by Berzelius in 1830. This phenomenon was first explained by A.M. Butlerov. The first monograph “About organic compounds isomery” by V.V. Markovnikov was published in 1865. Cycloalkanes isomerization was studied by V.V. Markovnikov, N.M. Kizhner and N.D. Zelinsky at the end of XIX century. First the alkylaromatic hydrocarbons isomerization reaction was described by Friedel and Crafts (1882), and catalytic butylene isomerization was described by V.N. Ipatyev in the beginning of XX century.Catalytic butane isomerization was described by Nenitescu and Dragan (1933) and also by B.L. Moldavsky.
Isomerization reactions are possible due to isomery – a phenomenon that consists in existing of several compounds with equal molecular weight, quantitative and qualitative composition, but with different physical and chemical characteristics. Such compounds are called isomers. For example, there are 5 basic hexane isomers, 3 conformation isomers of cyclohexane apart from methylcyclopentane, 17 hexene isomers. Octane has 18 isomers and tetradecane has as much as 1818 isomers.
There are two main types of isomery: structural and spatial (stereoisomery).
There are following types of isomerization in terms of hydrocarbons. N-butane to isobutane or m-xylene to p-xylene transforming can be an elementary example of carbon skeleton isomerization. Ring-chained isomerization, for example, propylene to cyclopropane or methylcyclopentane to cyclohexane, is a particular case of carbon skeleton isomerization. Butene-1 to cis-butene-2 can be an example of double bond position isomerization between carbon atoms. Cis-butene-2 to trans-butene-2 conversion illustrates an example of geometrical (spatial and configurational) isomerization. Cis-1,2-dimethylcyclopentane to trans-1,2-dimethylcyclopentane conversion can be referred to this isomerization type. One of the spatial isomery cases is stereoisomers occurrence, also called optical, that is differently rotating the polarized light plane, for example, 3-methylhexane. Even n-alkanes, despite their molecular structure is not linear but “zigzag”, can exist as rotational (conformational) isomers. Conformational isomerization is a result of atoms (group of atoms) rotation in a molecule around simple (ordinary C-C-bonds). For example, n-butane has 4 conformational isomers, of which the most energy stable form is transoid.
Isomerization reactions are used extensively for production of both lower and higher paraffins (isoalkanes).
Branched C5-C6 paraffins have high octane numbers and are good motor gasoline components.
Isopentane and isobutane are valuable feed for synthetic rubber production. Isobutane is also used for alkylbenzene, high-octane ethers production, methyl-tret-butyl ether (MTBE) is the most popular of those.
Higher alkanes isomerization favours decreasing of diesel fuel and engine oil pour point.
The following types of reaction are typical for isomerization process:
- paraffin isomerization;
- opening of naphthenic compounds rings;
- naphthenes isomerization;
- benzene saturation;
- hydrocracking
- naphthenes transalkylation.
Paraffin hydrocarbons isomerization reactions are equilibrium and proceed without volume change, therefore thermodynamic equilibrium depends only on temperature: low temperature favours creation of more branched isoparaffin hydrocarbons, however, isomerization rate increases in case of temperature rise.
There are other several important reactions besides paraffin isomerization reaction.
In the course of gasoline isomerization, rings opening reaction proceeds faster with temperature rise. For typical conditions in an isomerization unit reactor, at naphthene rings opening with creation of paraffin hydrocarbons conversion level is about 20-40%.
Naphthenic hydrocarbons methylcyclopentane and cyclohexane are at equilibrium.
In case of temperature rise, equilibrium shifts to methylcyclopentane formation side.
Benzene hydrogenation reaction runs very fast at very low temperatures with heat release. The amount of released heat limits benzene content in feed supplied to a unit. Feed supplied to the isomerization reactor block must contain no more than 1 wt.% of benzene.
Hydrocracking reaction is a side reaction. Conversion degree at hydrocracking depends on feed quality and rigidity of process operating mode. Molecules with big amount of carbon atoms, such as C7, are hydrocracked easier than molecules with lesser amount of carbon atoms. C5-C6 paraffins are also hydrocracked to some degree. In the result of hydrocracking reaction methane, ethane, propane and butane are formed.
Gasoline isomerization process depends on the following parameters:
- temperature;
- pressure;
- feed space velocity;
- molar correlation of hydrogen/crude and HBG circulation ratio;
- catalyst activity;
- feed composition and impurities content.
IMPORTANCE OF LIGHT NAPHTHA ISOMERIZATION UNITS
The isomerization process is one of the most profitable ways to produce high-octane components of gasoline with improved ecological characteristics. Isomerization units importance has also increased with introduction of new ultrahard limiting requirements for motor gasoline ecological characteristics including fractional composition, aromatic compounds and benzene content limitation. Isomerization units allow producing fuel with characteristics, complying with the strict standards EURO-4 and EURO-5. An intensive growth of isomerization process capacity is implemented due to of existing units revamp and building of grass-root units. Revamp and intensification of operating isomerization units to processes with recycle of unreacted normal paraffins are being done simultaneously. Light naphtha with EBP 62-85 oC is isomerization feed. Octane number increases due to increase of isoparaffins amount. As a rule, the process occurs in one or two reactors at a temperature 110-380 oC and the pressure up to 35 atm (depends on applied technology).
An isomerization unit is represented by a process system, consisting of blocks interconnected by process streams:
- feed pretreatment block (as a rule, includes feed hydrotreatment, hydrogenate stabilization in stripping column, and also may include adsorptive feed treatment over molecular sieves);
- precise isomerization feed fractionation block and/or isomerate;
- isomerization block (as a rule, includes directly reactor block and recycle gas drying section);
- stabilization block of the produced isomerate;
Hydrotreating process is a catalytic process, proceeding in hydrogen gas using specially matched catalyst. The purpose of the feed pre-hydrotreating process for the isomerization unit is removal of sunstances, deactivating catalyst. Sulfur, oxygen and nitrogen compounds; metalorganic compounds, containing arsenic, copper, etc. as well as unsaturated compounds refer to these substances.
Sometimes reforming units and isomerization units are unified to single high-octane gasoline production complex. Process scheme of a particular isomerization unit will depend on an isomerization catalyst type, which is planned to be charged into a reactor block.
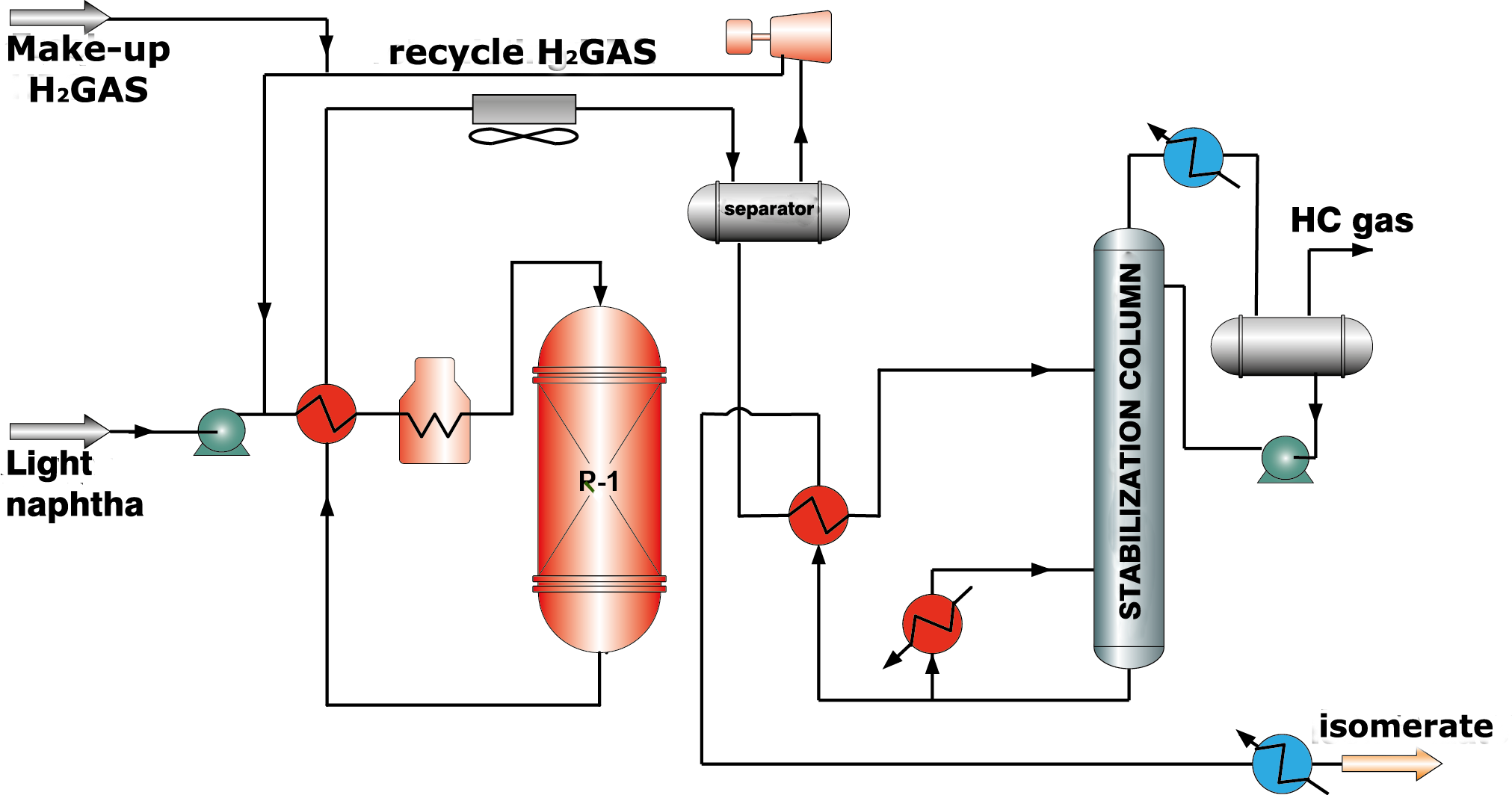
Zeolite catalysts display activity at higher temperatures comparing with catalysts of the other types and, therefore, low octane numbers of isomerate (76-78 RON). However, they have high resistance to poisons in feed and ability to regenerate in unit reactor completely. In the technological scheme of this process fire heaters for gas-feed mixture heating up to reaction temperature are provided. Sufficiently high hydrogen to hydrocarbon feed ratio is required (along with isomerization hydrogen is spent for feed dearomatization), therefore a compressor for recycle hydrogen gas supply and a separator for hydrogen gas separation are necessary (picture 1).
Picture 1. Isomerization process flow diagram over zeolite catalysts
Catalysts based on chlorinated alumina
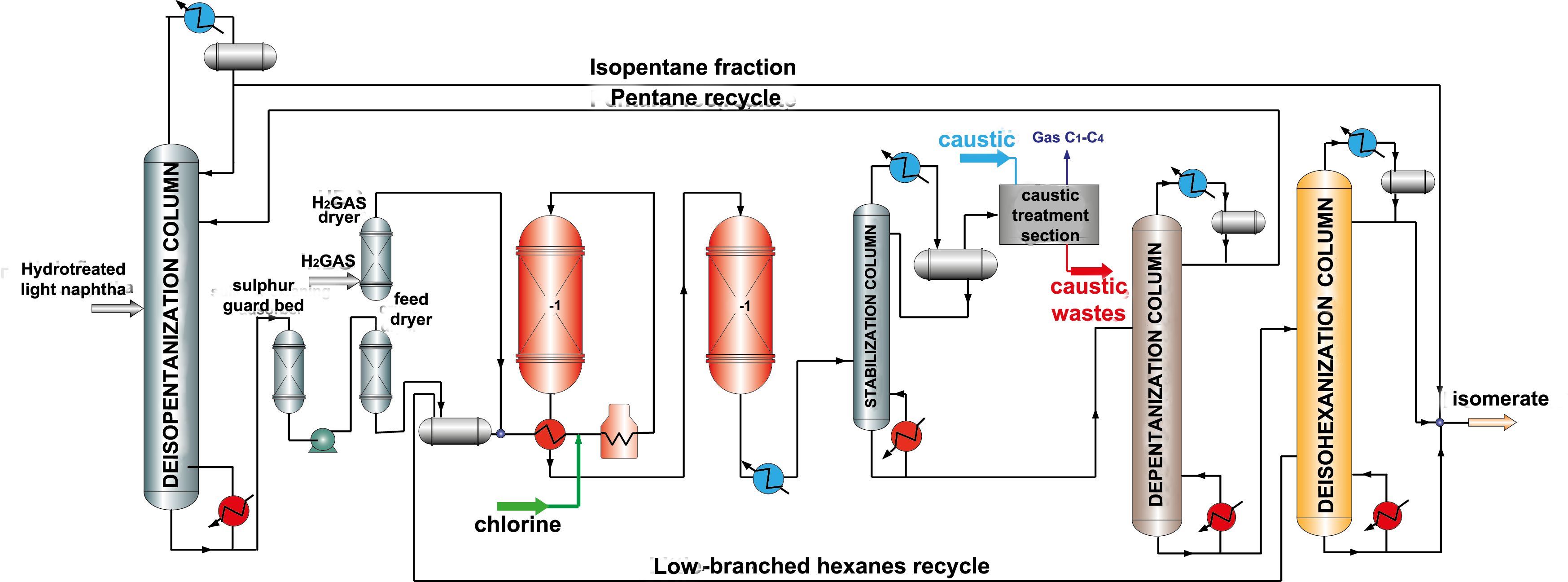
Catalysts based on chlorinated aluminum oxide are the most active and provide high yield and octane number of isomerate. It is necessary to say that during isomerization such catalysts loose chlorine, as a result activity decreases. Therefore, injection of chlorinated compounds (usually CCI4) into feed is provided to maintain high catalyst activity. After that caustic treatment to remove organic chlorine in special scrubbers is necessary. A big disadvantage is that this type of catalyst is very sensitive to catalytic poisons (oxygen-containing compounds, water, nitrogen, sulfur, metals) and requires very accurate feed preparation (picture 2). Chlorinated catalysts do not regenerate, and their service life is 3-5 years.
Picture 2. Isomerization process flow diagram over chlorinated catalysts with pentane and hexane recycle.
Catalysts based on sulfated metal oxide
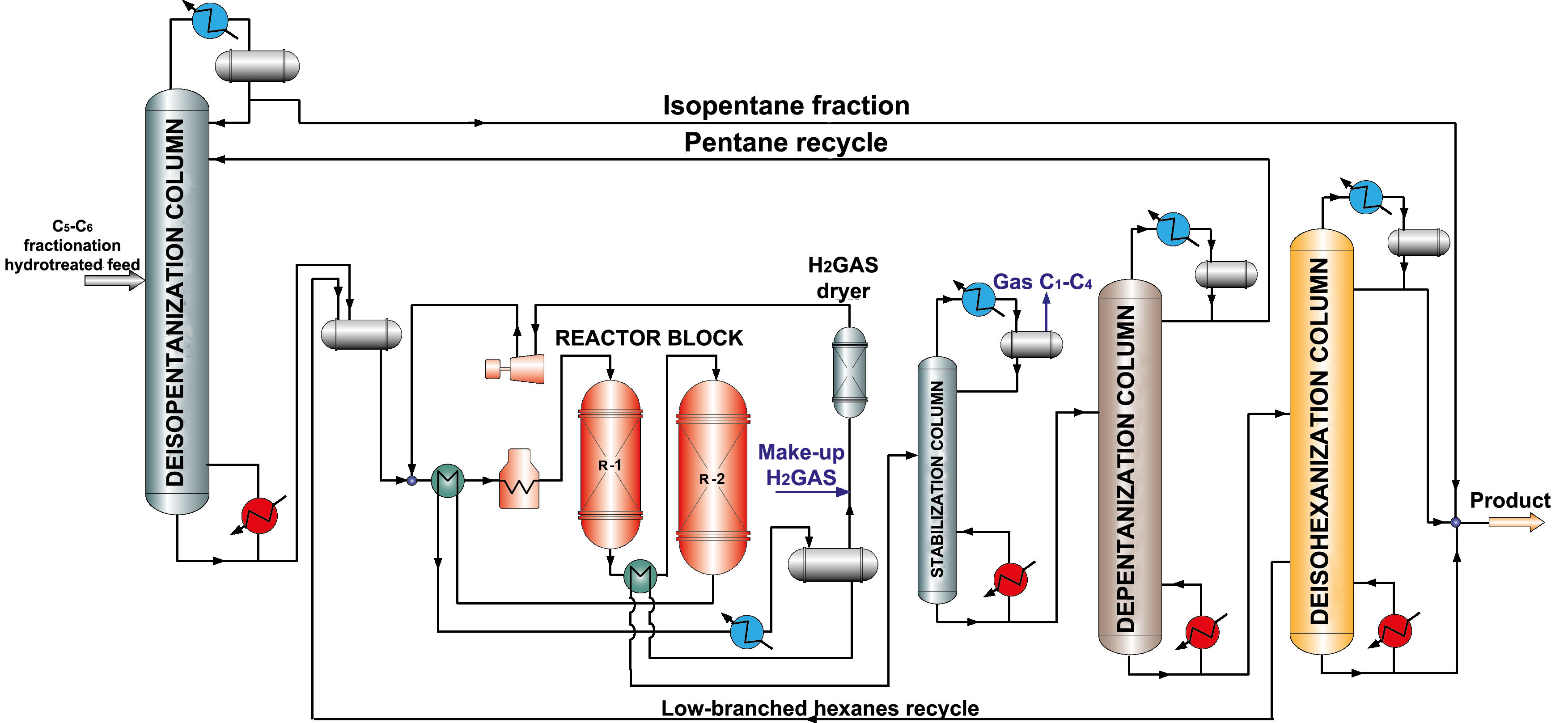
Catalysts based on sulfated metal oxide (oxide catalysts) have become popular recently, because they combine high activity and resist to catalytic poisons action, are able to regenerate. As well as for zeolite catalysts, there is a need for compressor to supply recycle hydrogen gas (picture 3), however there is no necessity in chlorine supply, adsorptive feed treatment and caustic treatment of hydrocarbon gas.
Picture 3. Isomerization process flow diagram over oxide catalysts with pentane and hexane recycle.
Information of this chapter is given exclusively for a reference purpose. You can find information about SIE Neftehim, LLC's products and services in Developments and Services chapters.










